Step-by-step explanation:
It is known that pH is negative log of
 . Also the relationship between pH and pOH is as follows.
. Also the relationship between pH and pOH is as follows.
pH + pOH = 14
3.75 + pOH = 14
pOH = 14 - 3.75
= 10.25
Therefore, pOH =
![-log [OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/college/r1588hr6xcow8p00ivmuenjgoj8x29z83e.png)
10.25 =
![-log [OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/college/r1588hr6xcow8p00ivmuenjgoj8x29z83e.png)
antilog 10.25 = -
![[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dbzut40quqceef230a4l1arx1nzd8511gj.png)
![[OH^(-)] = 5.6 * 10^(-11)](https://img.qammunity.org/2019/formulas/chemistry/college/4lwur2bcyhtld6vak8damv27vk1dtfhyqw.png)
Thus, we can conclude that
![[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dbzut40quqceef230a4l1arx1nzd8511gj.png) for the solution is
for the solution is
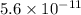 .
.