Answer:The value of the
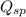 is
is
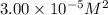 .
.
Step-by-step explanation:
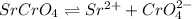
![[Sr^(2+)]=2.00* 10^(-2) M](https://img.qammunity.org/2019/formulas/chemistry/college/1itmwyy4ettzh3zsmu1x9hafif1f50w3j8.png)
![[CrO_4^(2-)]=1.50* 10^(-3)M](https://img.qammunity.org/2019/formulas/chemistry/college/7yjh645uzzl64zk2t8n8dpdfgrranwj3z2.png)
The
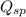 of the salt solution is defined as the product of of the concentration of the ions raised to power equal to their stoichiometric coefficient.
of the salt solution is defined as the product of of the concentration of the ions raised to power equal to their stoichiometric coefficient.
At equilibrium the value of
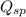 is equal to the value of
is equal to the value of
![K_{sp]](https://img.qammunity.org/2019/formulas/chemistry/college/4toi4pdjtdqiijah2yoxcjw8y98ge7athr.png) (solubility product).
(solubility product).
![Q_(sp)=[Sr^(2+)]^1* [CrO_4^(2-)]^1](https://img.qammunity.org/2019/formulas/chemistry/college/7zt314izsrcavx26meusfflpz2z718c58t.png)
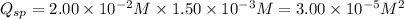
The value of the
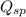 is
is
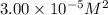 .
.