Hello!
1) What is the limiting reactant?The chemical equation for the reaction is the following:
Fe + S → FeS
To determine the limiting reactant, we will determine the
moles of each reactant and divide it by the coefficient in the chemical equation (In this case is I for each one)
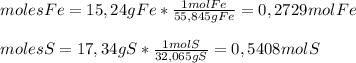
Now, the reactant with the less number of moles is Fe, so
Fe is the limiting reactant.
2) The reactant in excess.
From the chemical reaction:
Fe + S → FeS
The reactant in excess is the one that is in the
highest amount. To determine which is the reactant in excess we need to calculate the moles of each reactant and divide by the coefficient in the chemical reaction:
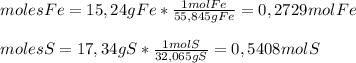
The reactant in excess is S, the amount that is left after the reaction is:
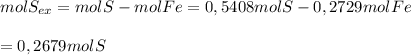
So there are
0,2679 moles of S in excess after the reaction.
3)
What mass of FeS is created?To determine the mass of FeS created we need to go from moles of Fe (Limiting reactant) to grams of FeS (Product) using the following conversion factor:
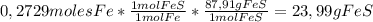
So,
23,99 grams of FeS are produced from this chemical reaction.
Have a nice day!