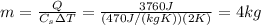The amount of heat needed to increase the temperature of a substance by

is:
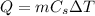
where
Q is the amount of heat
m is the mass of the substance
Cs is the specific heat capacity of the substance

is the variation of temperature
We know the heat added to the block of iron, Q=3760 J, its variation of temperature,
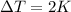
, and the specific heat capacity of iron,
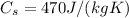
, so we can re-arrange the previous formula to find the mass of the block: