The correct answer is:
B) 0 J
Let's see why. The work done by the gas is equal to
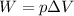
where
p is the pressure
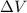
is the variation of volume of the gas.
However, the problem says that the volume is kept constant (2.0 L), so the variation of volume is zero:
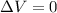
, and the work done is zero as well.