Answer:We will use an acid to test whether sample contains calcium carbonate or not.
Step-by-step explanation:
Calcium carbonate reacts with acids to give off carbon-dioxide gas ,water and calcium chloride. it is a salt of strong base calcium hydroxide and weak acid carbonic acid.
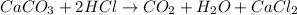
Evolution of carbon-dioxide on the reaction is also termed as effervescence.
If the sample contains the calcium carbonate strong effervesces will be observed and if not no effervescence will be observed.
We will use an acid to test whether sample contains calcium carbonate or not.