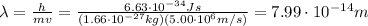De Broglie's identity gives the relationship between the momentum and the wavelength of a particle:
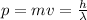
where
p is the particle momentum
m is its mass
v its velocity
h is the Planck constant
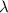
is the wavelength
By re-arranging the equation, we get
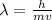
and by using the data about the proton, given in the text, we can find the proton's wavelength: