pH is calculated by using following formula,
pH = - log [H⁺]
As we are not given with [H⁺], so we will calculate it bu using following formula,
[H⁺] =
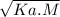
Putting Values and solving,
[H⁺] =
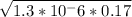
[H⁺] =
 [H⁺] = 4.70 × 10⁻⁴
[H⁺] = 4.70 × 10⁻⁴Now,
pH = - log (4.70 × 10⁻⁴)
pH = 3.32