Answer: Option (d) is the correct answer.
Step-by-step explanation:
When we dissolve an ionic compound in water then it readily dissociates into ions.
This is because ionic compounds are polar in nature and like dissolves like. Since, water is also polar in nature hence,
For example, NaF is an ionic compound as it is formed by transfer of an electron from sodium atom to fluorine atom.
So, when NaF is dissolved in water then it dissociates into
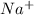 and
and
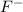 ions.
ions.
As electric current occurs due to movement of electrons or ions. Therefore, a solution with
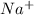 and
and
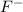 ions is able to conduct electricity.
ions is able to conduct electricity.
Thus, we can conclude that if you want to conduct an electrical current, then dissolving solid NaF in water would produce a solution capable of this.