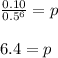6.4 micrograms were administered to the patient.
The general form of this equation would be:
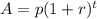
, where A is the total amount left, p is the initial amount, r is the rate of decay, and t is the number of time periods.
For our question, A =0.1 since that is the amount left; p is unknown; r is -0.5, since it loses half of its mass; and t will be 6. 2 hours = 120 minutes/20 minutes = 6 20-minute increments:
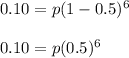
Divide both sides by 0.5^6: