Answer : Heat, Q = 526.68 J
Explanation :
Given that,
The mass of water, m = 7 g
Change in temperature,
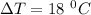
Heat required to raise the temperature is given by :
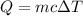
Where,
c is the specific heat of the water
For water,
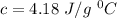
So,
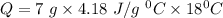
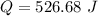
So, heat needed to raise the temperature of 7g of water by
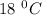 is 526.68 J
is 526.68 J
Hence, this is the required solution.