Answer: The concentration of H subscript two to the exponent 2 multiplied by the concentration of S2 in the numerator all over the concentration of H2S to the exponent 2 in the denominator.
Step-by-step explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients.
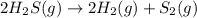
The equilibrium constant in terms of concentration is written as :
![K_c=([H_2]^2* [S_2])/([H_2S]^2)](https://img.qammunity.org/2019/formulas/chemistry/high-school/x6dlowc624vz3hrvzlet20cgsqqzwmt427.png)
The numerals written below the symbols are subscripts and the numerals above are called as exponents.
Thus the concentration of H subscript two to the exponent 2 multiplied by the concentration of
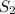 in the numerator all over the concentration of
in the numerator all over the concentration of
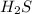 to the exponent 2 in the denominator.
to the exponent 2 in the denominator.