Answer: The correct answer is option(C).
Step-by-step explanation:
The balanced chemical equation of decomposition of hydrogen peroxide is given as:
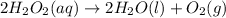
By stoichiometery of the reaction 2 moles of hydrogen peroxide on decomposition gives 2 mole of water and 1 mole of oxygen.
Hence,the correct answer is option(C).