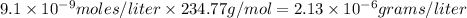Answer: Molar solubility is
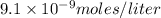 and solubility in grams per liter is
and solubility in grams per liter is
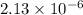
Explanation: The equation for the reaction will be as follows:
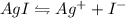
1 mole of
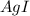 gives 1 mole of
gives 1 mole of
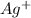 and 1 mole of
and 1 mole of
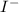 .
.
Thus if solubility of
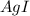 is s moles/liter, solubility of
is s moles/liter, solubility of
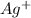 is s moles\liter and solubility of
is s moles\liter and solubility of
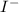 is s moles/liter.
is s moles/liter.
Therefore,
![K_sp=[Ag^+][I^-]](https://img.qammunity.org/2019/formulas/chemistry/college/nm3b40w13zxtpbprkgp0xmmnrmgfv8nd2d.png)
![8.3* 10^(-17)=[s][s]](https://img.qammunity.org/2019/formulas/chemistry/college/7p4nohg7kzcvzvw254oi8bjtqxszczrdkv.png)
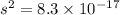

Solubility in grams/liter=
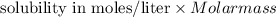
Solubility in grams/liter=