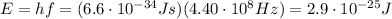The energy of a single photon is given by
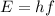
where
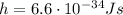
is the Planck constant
f is the frequency of the wave (of the photon)
In our problem, the radio wave has a frequency of
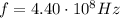
, so if we put this value into the previous formula, we can find the energy of a single photon of this electromagnetic wave: