Answer: Option (4) is the correct answer.
Step-by-step explanation:
When a solution contains number of ions then this type of solution is able to conduct electricity.
For example, when NaCl is dissolved in water the NaCl dissociates into
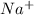 and
and
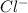 ions. Hence, flow of these ions will be responsible for electrical conductivity.
ions. Hence, flow of these ions will be responsible for electrical conductivity.
Whereas flow of electrons in a conductor is responsible for electrical conductivity but in a solution ions are responsible for the flow of electrical current.
Thus, we can conclude that the electrical conductivity of an aqueous solution depends on the concentration of which particles in the solution ions.