The balanced equation for the above double displacement reaction is as follows;
2KCl + Pb(NO₃)₂ ---> PbCl₂ + 2KNO₃
Stoichiometry of KCl to PbCl₂ is 2:1
This means that 2 mol of KCl would react with every 1 mol of PbCl₂
The molarity of KCl - 0.596 M
in 1 L of KCl there are 0.596 mol
Therefore in 10.0 mL of KCl there are -
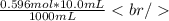
Number of KCl moles reacted - 0.00596 mol
according to stoichiometry
number of PbCl₂ moles formed - 1/2 x number of KCl moles reacted
Therefore number of PbCl₂ moles formed - 0.00596 mol/2 = 0.00298 mol
molar mass of PbCl₂ - 278 g/mol
mass of PbCl₂ formed - 278 g/mol x 0.00298 mol = 0.828 g