Answer : The number of moles of tin(IV)chloride formed are, 1.425 moles
Solution : Given,
Moles of chlorine gas = 2.85 moles
The balanced chemical reaction will be,
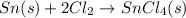
From the balanced reaction, we conclude that
As, 2 moles of chlorine gas react to give 1 mole of tin(IV)chloride,
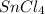
So, 2.85 moles of chlorine gas react to give
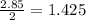 moles of tin(IV)chloride,
moles of tin(IV)chloride,
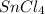
Hence, the number of moles of tin(IV)chloride formed are, 1.425 moles