Hello!
The general chemical equation for the neutralization of an acid HA with NaOH is the following:
HA(aq) + NaOH(aq) → NaA(aq) + H₂O(l)
For determining the concentration of the acid solution, we can use the equation shown below:
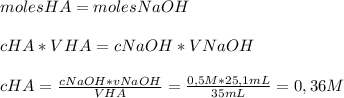
So, the concentration of the Acid is
0,36 MHave a nice day!