Answer : The number of valence electrons in fluorine are, seven.
Explanation :
As we know that the fluorine is a non-metal element that belongs to the group 17 and period 2. The symbol of fluorine element is, (F) and the atomic number of fluorine is, 9.
The electronic configuration of fluorine is,
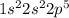
Electronic configuration : It is defined as the distribution of electrons of an atom in an atomic orbitals.
From the electronic configuration, we conclude that the number of valence electrons present in fluorine is, seven (7).
Hence, the number of valence electrons in fluorine are, seven.