Answer: The correct answer is Option B.
Step-by-step explanation:
Kinetic energy is defined as the energy which is possessed by the motion of a particle. The formula for the kinetic energy follows:
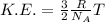
where, R = Gas Constant
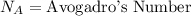
T = Temperature
From above expression, kinetic energy is directly proportional to temperature of the system
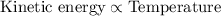
As the temperature is increases, the kinetic energy of the particles also increases and vice-versa.
In the given question,
The gas (carbon-dioxide) is heated which means the temperature of the gas is increases and hence, the kinetic energy will also be increased.
Hence, the correct answer is Option B.