Answer: 0.08585 moles of
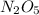 is needed to produce 7.9 grams of
is needed to produce 7.9 grams of

Explanation: Moles is calculated by using the formula:
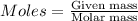
Molar mass of
 = 46 g/mol
= 46 g/mol
Number of moles of
 = 0.1717 moles
= 0.1717 moles
For the given chemical reaction:
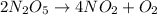
By Stoichiometry,
4 moles of
 is produced by 2 moles of
is produced by 2 moles of
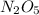
So, 0.1717 moles of
 is produced by =
is produced by =
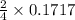 = 0.08585 moles of
= 0.08585 moles of
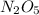
Hence, moles of
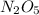 is 0.08585 moles.
is 0.08585 moles.