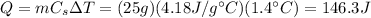The amount of heat released by the pebble is equal to the amount of heat absorbed by the water, which is given by
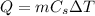
where
m is the mass of the water
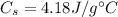
is the specific heat capacity of the water
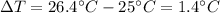
is the increase in temperature of the water.
The density of the water is
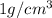
, and
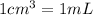
, so the mass of the water in the problem is
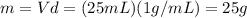
so if we substitute in the formula, we get the amount of heat absorbed by the water (and released by the pebble):