I am attempting the problem for phosphonium Ion rather than its chloride salt. The chemical equation is shown below along with molar masses in mg.
First of all we will calculate the amounts of reactants required for the synthesis of 220 mg of phophonium ion. Calculations for both reactants is as follow,
For
Benzyl chloride,
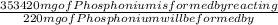
=
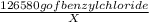
Solving for X,
X =
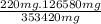
X = 78.79 mg
For PPh₃:
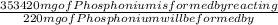
=
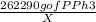
Solving for X,
X =
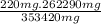
X = 163.27 mg
Now, Assuming these values as for 95 % conversion, we can calculate 100 % yield as follow,
when
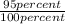
=
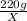
Solving for X,
X =
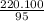
= 231.57 mg
Now, calculate reactants mass with respect to 231.57 mg
when
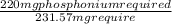
=
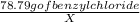
Solving for ,
X =
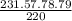
=
82.93 mg of Benzyl chloride
when
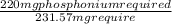
=
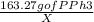
Solving for ,
X =
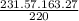
=
171.85 mg of PPh3
So, reaction was started with reacting
82.93 mg of Benzyl Chloride and
171.85 mg of Triphenyl Phosphine.