Hello!
The mass of CO that was used up in the reaction with an excess of oxygen gas if 24,7 g of Carbon Dioxide is formed was
15,72 gThe chemical equation for the reaction in this exercise is the following one:
2 CO + O₂ → 2CO₂
Now, we will apply the following conversion factor to go from grams of Carbon Dioxide to grams of Carbon monoxide using the molar masses, and reaction coefficients:
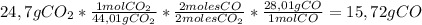
Have a nice day!