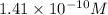Answer : The hydronium ion concentration in an aqueous solution is,
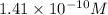
Explanation : Given,
pH = 9.85
pH : It is defined as the negative logarithm of hydronium or hydrogen ion concentration.
Formula used :
![pH=-\log [H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/college/kv1d6x7k5u8rrmnok6mdbtm4oardzajzce.png)
Now put the value of pH in this formula, we get:
![9.85=-\log [H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/college/ffl8o5feyqsj80mjj548tffbnvb6yt9gpg.png)
![[H_3O^+]=1.41* 10^(-10)M](https://img.qammunity.org/2019/formulas/chemistry/college/pzhncj9xgt8t8nv02ei0iisfsp2dmxlaem.png)
Therefore, the hydronium ion concentration in an aqueous solution is,