Answer:
the total number of its protons and neutrons
Step-by-step explanation:
An atom is represented by
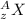
here we know that
z = atomic number
it represent the total number of protons or total number of electrons present in an neutral atom
A = mass number
it represents the total number of neutrons and protons present inside the nuclei of an atom
this is also known as atomic mass of an atom in amu (atomic mass unit)