Formula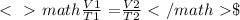 Givens
Givens
V1 = 595 mL
T1 = 24.7° C = 24.7° + 273 = 297.7° K
V2 = 876 mL
T2 = ???
Substitution and Solve595 / 297.7 = 876 / x Cross multiply
595*x = 297.7 * 876
x = 297.7 * 876 / 595
x = 260785 /595
x = 438 degrees Kelvin
T2 = 438 degrees Kelvin
D <<<< answer