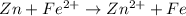Answer:
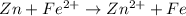
Explanation:
Oxidation reaction : A reaction in which electrons are lost and there is an increase in oxidation state.
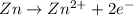
Reduction reaction : A reaction in which electrons are gained and there is a decrease in oxidation state number.
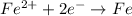
A redox reaction is one in which oxidation and reduction takes place in a single reaction.