To answer this question we must at the beginning write a balanced chemical equation:
Ag₂CrO₄ ↔ 2 Ag⁺ + CrO₄²⁻
As we see from the equation that ionization of one mole of silver chromate gives two moles of silver and one mole of chromate
So the solubility product Ksp = [Ag⁺]²[CrO₄⁻²]
[CrO₄⁻²] = 6.7 x 10⁻⁵ M and Ksp = 1.2 x 10⁻¹² (as given)
So:
1.2 x 10⁻¹² = [Ag⁺]² (6.7 x 10⁻⁵)
[Ag⁺]² =
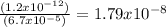
![[Ag^(+)] = \sqrt{1.79 x 10^(-8)} = 1.35 x 10^(-4)](https://img.qammunity.org/2019/formulas/chemistry/college/xcxn903jkvknmz7gb6rvi1elr7vehgttfq.png)
[Ag⁺] = 1.34 x 10⁻⁴ M