Ni(OH)₂ ⇄ Ni⁺² + 2 OH⁻
Ksp = [Ni⁺²][OH⁻]² = S (2S)² = 4S³
where S is molar solubility.
at pH = 10
[H⁺] = 10⁻¹⁰
[H⁺][OH⁻] = 10⁻¹⁴
so [OH⁻] = 10⁻⁴ M
Ksp = S [10⁻⁴ + 2S]²
Ksp is very small so the molar solubility of OH⁻ will be very small
so (10⁻⁴ + 2S) is about 10⁻⁴
so Ksp = S x 10⁻⁸
S =
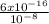
= 6 x 10⁻⁸ M