Answer:
Explanation: Ionization energy is the removal of an electron from the outermost shell of the gaseous atom.
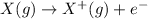
When we remove one electron, the species acquire a positive charge because the number of protons has been increased than the number of electrons . In reference to this, the nucleus would hold its grip to the valence shell more than before due to the electrostatic forces of attraction.
Thus, due to this electrostatic forces of attraction, it will become difficult to remove an electron from the last shell.
Thus ionization energy will increase on removal of successive electrons .