Gay-Lussacs law relates the pressure of gas with temperature when all the other conditions are kept constant.
It states that for a fixed amount of gas, pressure of gas is directly proportional to temperature when the volume is constant.
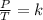
where P - pressure, T- temperature and k - constant
temperature has been given in Celsius, we need to convert to Kelvin
Temperature in K = Temperature in Celsius + 273.15
1st instance T1 = 35 °C + 273.15 = 308.15 K
P1 = 97 kPa
2nd instance T2 = 75 °C + 273.15 = 348.15 K
P2 - needs to be calculated using following equation
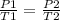
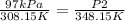
P2 = (97 x 348.15)/308.15
P2 = 110.0 kPa