The answer is "the specific heat of the substance".
In fact, specific heat is a property of every substance that tells how much heat is needed to raise the temperature of a certain amount of that substance by 1 degree.
Using formula:
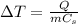
where

is the increase in temperature of the substance, Q is the amount of heat absorbed by the substance, m is its mass and Cs is the specific heat of that substance.
In the problem, the mass of the water and of the wood is the same (1 kg), and they absorb the same amount of heat, Q. But the change in temperature

of the wood is larger than the water, and the explanation for this is that the specific heat Cs of the wood is smaller than the specific heat of water.
In fact, looking at tables we have that specific heat for water is 1 J/(g C), while for wood is approximately 0.4 J/(g C).