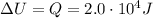The change in internal energy of the gas is
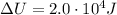
.
In fact, the 1st law of thermodynamics states that the change in internal energy of a system is equal to the amount of heat given to the system (Q) plus the work done on the system (W):
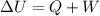
In this example, no work is done on the bottle so W=0, while the heat given to the system is
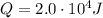
, so the change in internal energy of the gas is