Answer:
Dipole-dipole forces are weak electrostatic attractions between the positive dipole of one polar molecule and the negative dipole of another.
Step-by-step explanation:
An example of such forces occurs between the hydrochloric acid molecules.
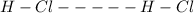
Dipole-dipole forces are represented with dotted lines.