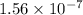Answer : The value of
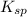 is
is
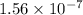
Explanation :
First we have to calculate the mass of CuCl in 1 L or 1000 mL solution.
As, 100.0 mL of solution contains 3.91 mg of CuCl
So, 1000 mL of solution contains
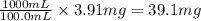 of CuCl
of CuCl
The mass of CuCl = 39.1 mg = 0.0391 g
conversion used : (1 mg = 0.001 g)
Now we have to calculate the moles of CuCl.
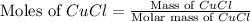
Molar mass of CuCl = 99.00 g/mol
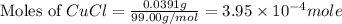
Now we have to calculate the moles of
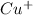 and
and
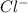 ion.
ion.
Moles of
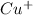 = Moles of
= Moles of
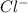 = Moles of CuCl =
= Moles of CuCl =
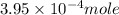
Thus, the concentration of
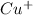 and
and
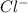 ion in 1 L solution will be:
ion in 1 L solution will be:
![[Cu^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/63li0i3p6u1b75rf08d71aj2s7z70heak9.png) =
=
![[Cl^-]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dbp2oxifgj5unfb1j0f7ctcdupm750e9f9.png) =
=
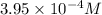
Now we have to calculate the value of
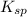 for CuCl.
for CuCl.
The solubility equilibrium reaction will be:
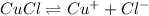
The expression for solubility constant for this reaction will be,
![K_(sp)=[Cu^(+)][Cl^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/y7g7r55m31hy3snn72to6ngh2mr8cbd22a.png)
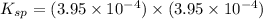
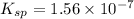
Therefore, the value of
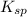 is
is