Answer: 2.84 moles
Explanation: Heat of solution is the heat change associated with the dissolution of a substance in a solvent at constant pressure.
The enthalpy of solution is most often expressed in kJ/mol at constant temperature.
It is given that: Molar heat of solutionof
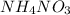 = 25.7 kJ/mol
= 25.7 kJ/mol
25.7 kJ of heat is absorbed when 1 mole of
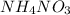 is dissolved in water.
is dissolved in water.
73.0 kJ is released when=
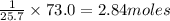 are dissolved in water.
are dissolved in water.