Answer : The correct option is, (C)the number of molecules
Explanation :
When we are comparing 1 mole of oxygen gas
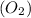 with 1 mole of carbon monoxide gas
with 1 mole of carbon monoxide gas
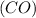 then we conclude that,
then we conclude that,
In 1 mole of oxygen gas
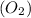 , there are two number of molecules of oxygen.
, there are two number of molecules of oxygen.
In 1 mole of carbon monoxide gas
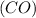 , there are two number of molecules (carbon and oxygen).
, there are two number of molecules (carbon and oxygen).
Hence, the number of molecules must be the same when comparing 1 mole of oxygen gas, with 1 mole of carbon monoxide gas.