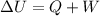Answer: The formula used to solve the problems related to first law of thermodynamics is
Step-by-step explanation:
First law of thermodynamics states that the total energy of the system remains conserved. Energy can neither be destroyed, nor be created but it can only be transformed into one form to another.
Its implication is any change in the internal energy will be either due to heat energy or work energy.
Mathematically,
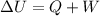
where, Q = heat energy
W = work energy
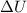 = Change in internal energy
= Change in internal energy
Sign convention for these energies:
For Q: Heat absorbed will be positive and heat released will be negative.
For W: Work done by the system is negative and work done on the system is positive.
For
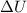 : When negative, internal energy is decreasing and when positive, internal energy is increasing.
: When negative, internal energy is decreasing and when positive, internal energy is increasing.
Hence, the formula used to solve the problems related to first law of thermodynamics is