Answer: Option (d) is the correct answer.
Step-by-step explanation:
A formula unit is basically empirical formula of a compound which tells us how many atoms of one element are combining with how many atoms of another element.
For example, empirical formula of sodium chloride is NaCl. So, basically it shows that one atom of sodium is reacting with one atom of chlorine.
Also according to Avogadro, there are
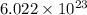 atoms present in 1 mole of each substance.
atoms present in 1 mole of each substance.
Thus, we can conclude that both are made up of
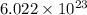 formula units best compares 1 mol of sodium chloride to 1 mol of aluminum chloride.
formula units best compares 1 mol of sodium chloride to 1 mol of aluminum chloride.