Answer : This is an example of single-displacement reaction.
Explanation :
Single-displacement reaction : It is a type of reaction in which the more reactive metal displaces the least reactive metal from its solution.
It is represented as :
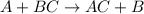
where A is the most reactive metal and BC is the compound in which the B is the least reactive metal which is displaced by the most reactive metal A.
The given balanced reaction is,
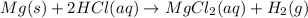
This reaction is an example of single-displacement reaction in which the magnesium metal displaces the hydrogen metal from its solution to give magnesium chloride and hydrogen gas as a product.
Hence, the given reaction is an example of single-displacement reaction.