Answer:
There are
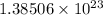 of bromine molecules present in the flask.
of bromine molecules present in the flask.
Step-by-step explanation:
Moles of liquid bromine in the flask = 0.230 mol
As we know that 1 mole is equal to the Avogadro number.
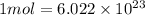 atoms/ molecules
atoms/ molecules
So, number of bromine molecules in 0.230 moles of liquid bromine is:
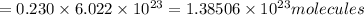
There are
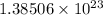 of bromine molecules present in the flask.
of bromine molecules present in the flask.