Answer: B.
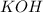
Explanation:
According to the Arrhenius concept, an acid is a substance that ionizes in the water to give hydronium ion or hydrogen ion
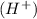 and a bases is a substance that ionizes in the water to give hydroxide ion
and a bases is a substance that ionizes in the water to give hydroxide ion
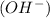 .
.
According to the Bronsted Lowry conjugate acid-base theory, an acid is defined as a substance which donates protons and a base is defined as a substance which accepts protons.
According to the Lewis concept, an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs.
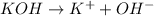 : is a Arrhenius base.
: is a Arrhenius base.
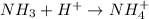 : is a lewis base as it donates the lone pair of electrons.
: is a lewis base as it donates the lone pair of electrons.
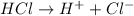 is a Arrhenius acid and bronsted lowry acid.
is a Arrhenius acid and bronsted lowry acid.