Answer:
- Both of their valence electrons are at p subshell.
- They have the first subshell full of electrons.
- Both of them have just 1 electron at the last p subshell.
Step-by-step explanation:
Hello,
In this case, we could understand their electron structures by identifying their electron configurations as shown below:
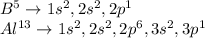
In such a way, we could notice the following similarities:
- Both of their valence electrons are at p subshell.
- They have the first subshell full of electrons.
- Both of them have just 1 electron at the last p subshell.
Best regards.