Answer:
The half-life (in seconds) of a zero-order reaction is 8.15 seconds.
Step-by-step explanation:
Initial concentration of the of the reactant =
![[A_o]=0.884 M](https://img.qammunity.org/2019/formulas/chemistry/college/sxy08zbykq4gqg7anpybf4bts5nt86dzyb.png)
The value of rate constant =
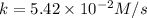
The half life for zero order reaction is given as:
![t-{(1)/(2)}=([A_o])/(2k)=(0.884 M)/(2* 5.42* 10^(-2) M/s)=8.15 s](https://img.qammunity.org/2019/formulas/chemistry/college/3dtx8xq1ncmwpncwpb6rxtivfhffjfo8dl.png)
The half-life (in seconds) of a zero-order reaction is 8.15 seconds.