Step-by-step explanation:
When iron reacts with the moisture present in air then an oxide layer tends to develop on the surface of iron. This process is known as rusting of iron.
Chemical formula of rust is
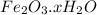 . This layer of oxide on the surface of iron is orange-brown in color.
. This layer of oxide on the surface of iron is orange-brown in color.
This change is a chemical change as a new compound iron oxide has formed in this process of rusting.
Hence, this orange-brown color on the surface of iron helps us to know that rusting has taken place.