Answer:
295.3744 grams of NaBr will produce 244 grams of
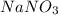 .
.
Step-by-step explanation:
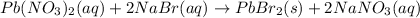
Moles of sodium nitarte=
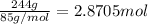
According to reaction, 2 moles of sodium nitrate is obtained from 2 moles of sodium bromide.
Then 2.8705 mol of sodium nitrate will be obtained from :
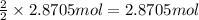 of sodium nitrate
of sodium nitrate
Mass of 2.8705 moles of sodium nitrate:
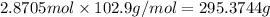
295.3744 grams of NaBr will produce 244 grams of
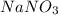 .
.