Answer: Option (c) is the correct answer.
Step-by-step explanation:
The given reaction equation is as follows.
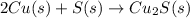
The oxidation-reduction half reactions will be as follows.
Oxidation :
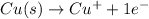
Reduction:
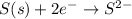
By balancing the above half reactions, oxidation-half reaction will be as follows.
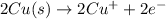
Whereas reduction-half reaction will remain the same.
Hence, it shows that each copper atom is losing one electron to combine with a sulfur atom.
Therefore, we can conclude that each copper atom loses one (1) electron.