Answer:
1.8 x 10²³molecules
Step-by-step explanation:
Given parameters:
Mass of H₂ = 8.95 x 10⁻⁴g
Unknown:
Number of molecules of NH₃ = ?
The equation of the reaction: N₂ + 3H₂ ⇄ 2NH₃
To solve this problem:
- We work from the known to the unknown. We know the mass of the hydrogen gas given so we find the number of moles using the equation below:
Number of moles of H₂ =
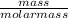
Number of moles =
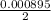
Number of moles = 0.00045mol
- From the balanced reaction equation, we can determine the number of moles of NH₃ produced:
3 moles of H₂ produced 2 moles of NH₃
0.00045mol of H₂ yields 0.000299mol of NH₃
To find the number of molecules of NH₃ produced, we use the expression below:
Number of molecules = number of moles x 6.02 x 10²³
Number of molecules of NH₃ = 0.000299 x 6.02 x 10²³ =
1.8 x 10²³molecules